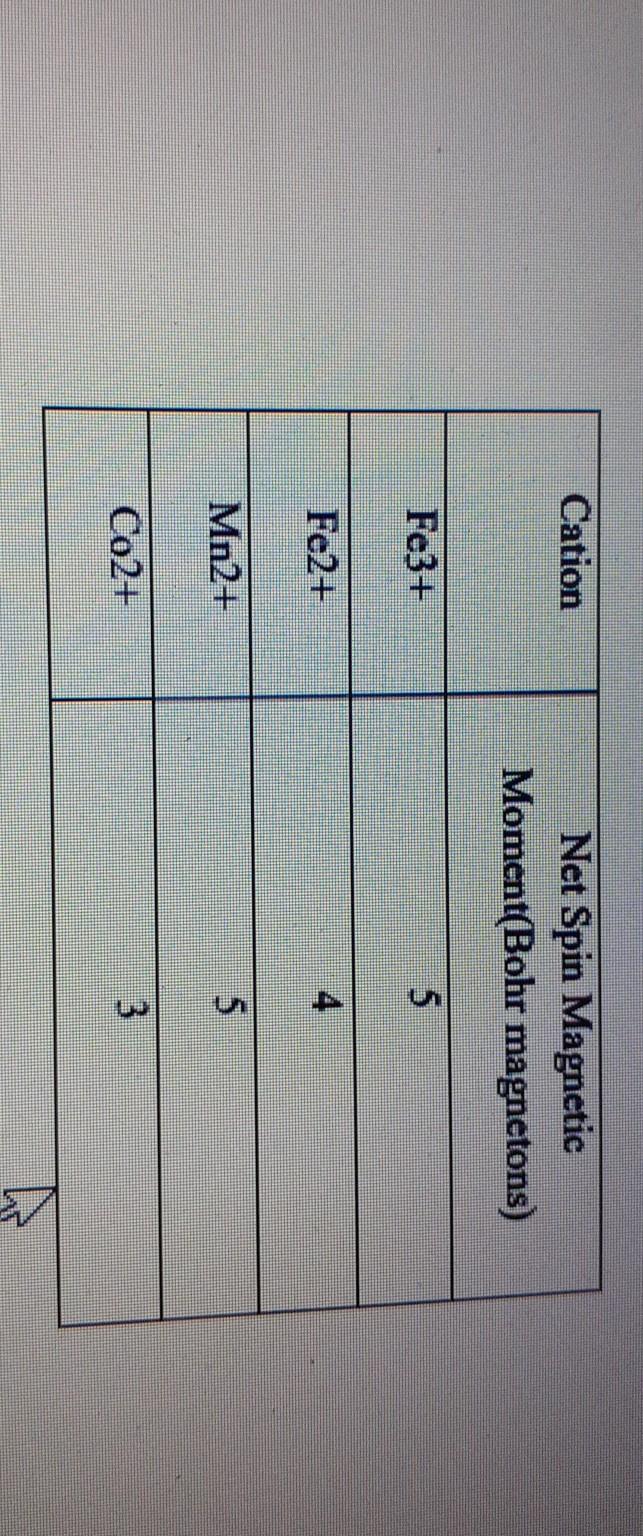
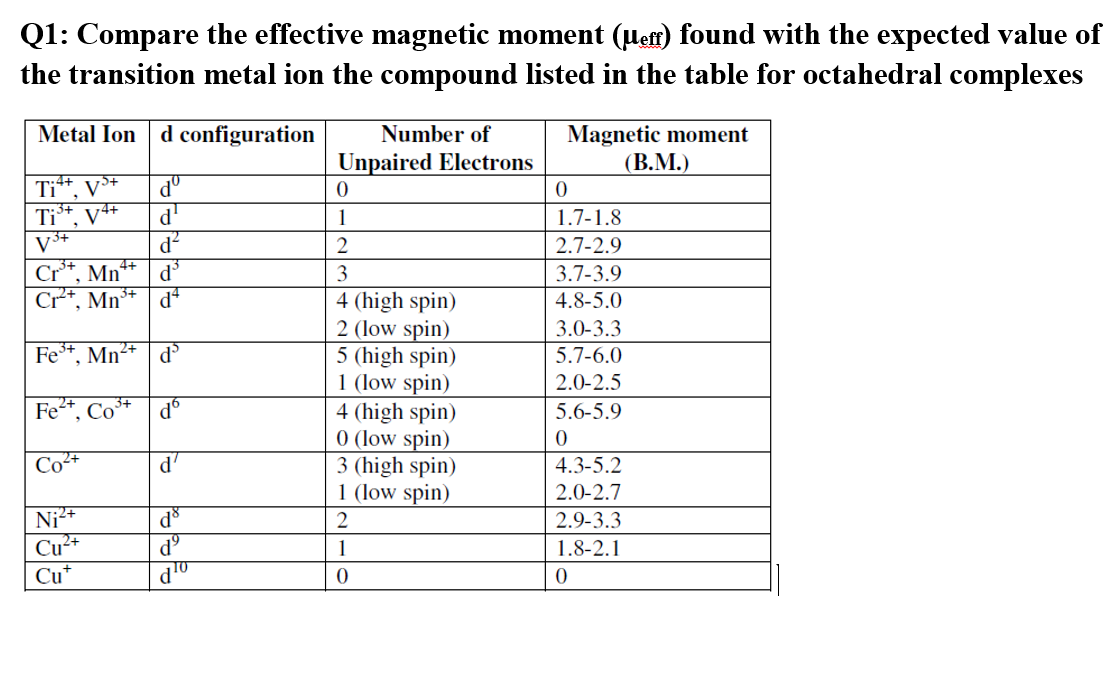
The spin only magnetic moment of Tetrachloridomanganate(II)ion is 5.9 BM. On the basis of VBT, - Sarthaks eConnect | Largest Online Education Community

Magnetic moment of `Cr^(+2)(Z=24),Mn^(+2)(Z=25)` and `Fe^(2+) (Z=26)` are x,y,z. they are in order - YouTube
Calculate the 'spin only' magnetic moment of M2+ (aq) ion (Z = 27). - Sarthaks eConnect | Largest Online Education Community

Among V(Z = 23) , Cr(Z = 24) , Mn(Z = 25) and Fe(Z = 26) , which will have the highest magnetic moment?

calculate the number of unpaired electrons in Ti3+ , Mn2+ and calculate the spin only magnetic - Brainly.in
What is the spin only magnetic moment of Cu+ ion? In my book it's given as root 2? What's the answer? - Quora
![The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` Pr... - YouTube The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` Pr... - YouTube](https://i.ytimg.com/vi/E3pLlLoxf48/maxresdefault.jpg)
The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` Pr... - YouTube

Choose the correct answer from the alternatives given :A sum of money is divided among A, B, C and D in the ratio of 3 : 7 : 9 : 13 respectively.

In the fallowing reaction: Cr2O7^2 - (aq) + SO3^2 - (aq) + 8H^+→ 2Cr^3 + + SO4^2 - + H2O the stoichiometric coefficient of SO3^2 - is:

Mn2+–Mn2+ Magnetic Coupling Effect on Photoluminescence Revealed by Photomagnetism in CsMnCl3 | The Journal of Physical Chemistry Letters


![Expert Answer] Calculate the spin only magnetic moment of mn2+ ion - Brainly.in Expert Answer] Calculate the spin only magnetic moment of mn2+ ion - Brainly.in](https://hi-static.z-dn.net/files/dc8/652c5b211abb681a4d61fb6a49aa9ae7.png)